期刊及DOI号
doi: 10.1186/s12943-021-01453-0.
CircDOCK1 promotes the tumorigenesis and cisplatin resistance of osteogenic sarcoma via the miR-339-3p/IGF1R axis
方法:Differentially expressed circRNAs in OS cell lines and tissues were identified by circRNA microarray analysis and quantitative real-time PCR (qRT–PCR). To explore the actions of circDOCK1 in vivo and in vitro, circDOCK1 was knocked down or overexpressed. To assess the binding and regulatory associations among miR-339-3p, circDOCK1 and IGF1R, we performed rescue experiments, RNA immunoprecipitation (RIP), RNA pulldown assays and dual-lucif-erase assays. Moreover, we performed apoptosis assays to reveal the regulatory effects of the circDOCK1/miR-339-3p/IGF1R axis on cisplatin sensitivity.
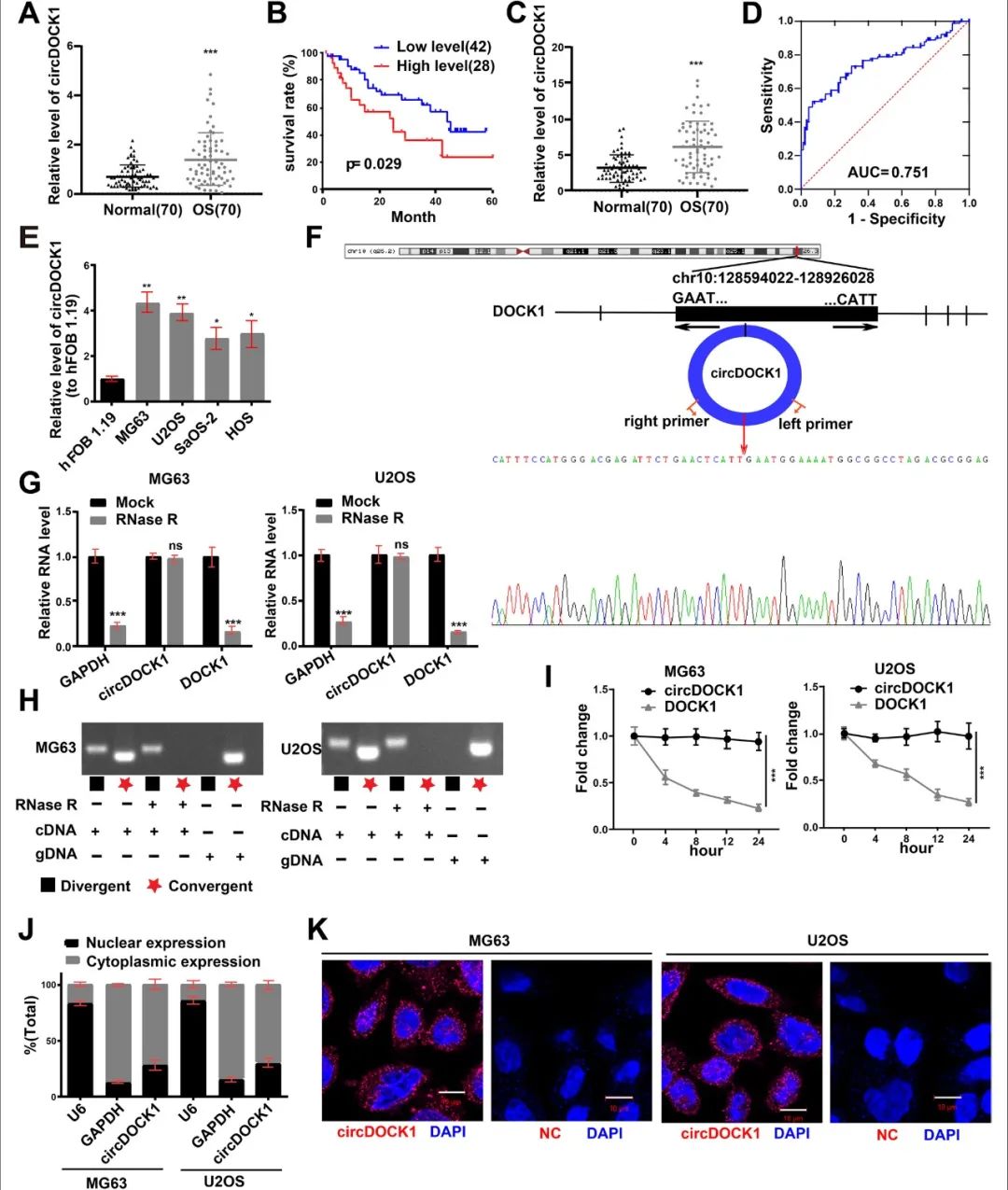
结果:CircDOCK1 expression remained stable in the cytoplasm and was higher in OS tissues and cells than in the corresponding controls. Overexpression of circDOCK1 increased oncogenicity in vivo and malignant transforma-tion in vitro. In the U2OS and MG63 cell lines, circDOCK1 modulated tumor progression by regulating IGF1R through sponging of miR-339-3p. Additionally, in the U2OS/DDP and MG63/DDP cell lines, cisplatin sensitivity was regulated by circDOCK1 via the miR-339-3p/IGF1R axis.
结论:CircDOCK1 can promote progression and regulate cisplatin sensitivity in OS via the miR-339-3p/IGF1R axis. Thus, the circDOCK1/miR-339-3p/IGF1R axis may be a key mechanism and therapeutic target in OS.
关键词:circDOCK1, miR-339-3p, IGF1R, OS, Cisplatin resistance

