J Exp Clin Cancer Res. 2022 Jan 3.
doi: 10.1186/s13046-021-02201-4.
题目
Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARα-CYP4X1 axis in colonic macrophages
背景:Immune checkpoint inhibitor-related cardiotoxicity is one of the most lethal adverse effects, and thus, the identification of underlying mechanisms for developing strategies to overcome it has clinical importance. This study aimed to investigate whether microbiota-host interactions contribute to PD-1/PD-L1 inhibitor-related cardiotoxicity.
方法:A mouse model of immune checkpoint inhibitor-related cardiotoxicity was constructed by PD-1/PD-L1 inhibitor BMS-1 (5 and 10 mg/kg), and cardiomyocyte apoptosis and cardiotoxicity were determined by hematoxylin and eosin, Masson’s trichome and TUNEL assays. 16S rRNA sequencing was used to define the gut microbiota composition. Gut microbiota metabolites short-chain fatty acids (SCFAs) were determined by HPLC. The serum levels of myocardial enzymes (creatine kinase, aspartate transaminase, creatine kinase-MB and lactate dehydrogenase) and the production of M1 factors (TNF-α and IL-1β) were measured by ELISA. The colonic macrophage phenotype was measured by mmunofluorescence and qPCR. The expression of Claudin-1, Occludin, ZO-1 and p-p65 was measured by western blot. The gene expression of peroxisome proliferator-activated receptor α (PPARα) and cytochrome P450 (CYP) 4X1 was determined using qPCR. Statistical analyses were performed using Student’s t-test for two-group com-parisons, and one-way ANOVA followed by Student–Newman–Keul test for multiple-group comparisons.
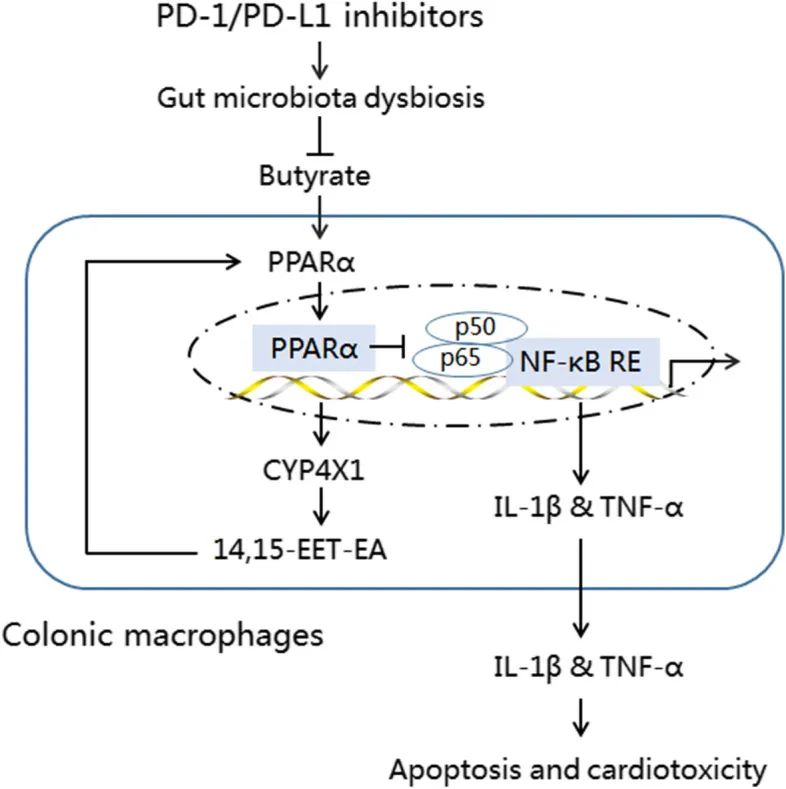
结果:We observed intestinal barrier injury and gut microbiota dysbiosis characterized by Prevotellaceae and Rikenellaceae genus depletion and Escherichia-Shigella and Ruminococcaceae genus enrichment, accompanied by low butyrate production and M1-like polarization of colonic macrophages in BMS-1 (5 and 10 mg/kg)-induced cardiotoxicity. Fecal microbiota transplantation mirrored the effect of BMS-1 on cardiomyocyte apoptosis and cardiotoxicity, while macrophage depletion and neutralization of TNF-α and IL-1β greatly attenuated BMS-1-induced cardiotoxicity. Importantly, Prevotella loescheii recolonization and butyrate supplementation alleviated PD-1/PD-L1 inhibitor- relared cardiotoxicity. Mechanistically, gut microbiota dysbiosis promoted M1-like polarization of colonic macrophages and the production of proinflammatory factors TNF-α and IL-1β through downregulation of PPARα-CYP4X1 axis.
结论:Intestinal barrier dysfunction amplifies PD-1/PD-L1 inhibitor-related cardiotoxicity by upregulating proinflammatory factors TNF-α and IL-1β in colonic macrophages via downregulation of butyrate-PPARα-CYP4X1 axis. Thus, targeting gut microbiota to polarize colonic macrophages away from the M1-like phenotype could provide a potential therapeutic strategy for PD-1/PD-L1 inhibitor-related cardiotoxicity.
关键词:PD-1/PD-L1 inhibitors, Cardiotoxicity, Gut microbiota, The colonic macrophage, CYP4X1

