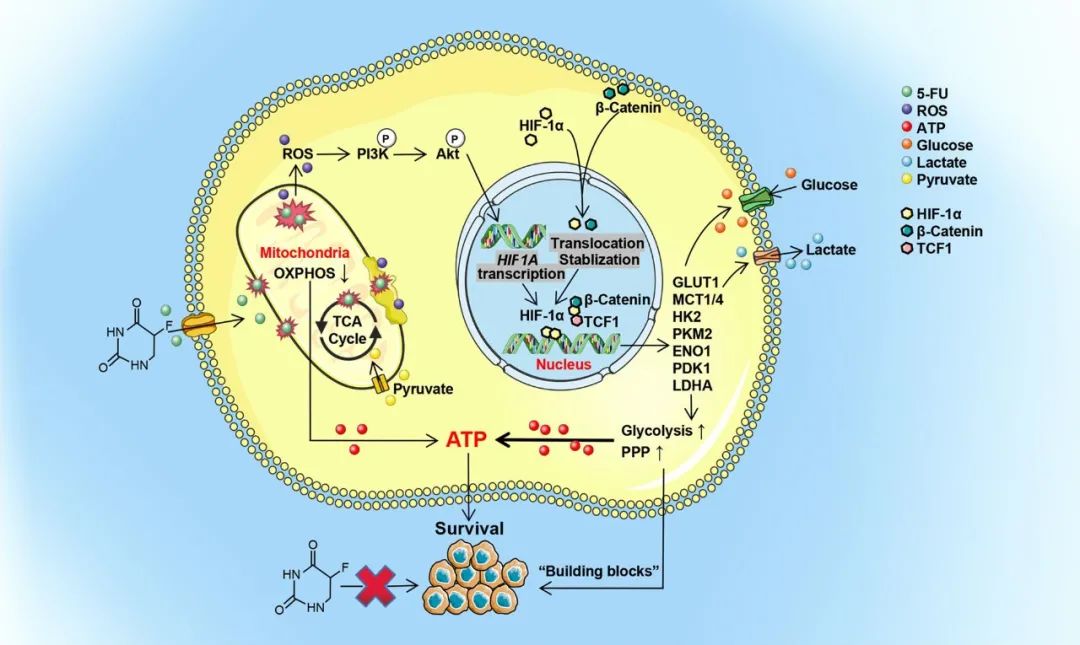
图 HIF-1α诱导葡萄糖代谢重编程在CRC中赋予5-FU耐药性的示意图。
J Exp Clin Cancer Res. 2022 Jan 8.
doi: 10.1186/s13046-021-02229-6.
题目
ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer
背景:Acquired resistance of 5-fluorouracil (5-FU) remains a clinical challenge in colorectal cancer (CRC), and efforts to develop targeted agents to reduce resistance have not yielded success. Metabolic reprogramming is a key cancer hallmark and confers several tumor phenotypes including chemoresistance. Glucose metabolic reprogramming events of 5-FU resistance in CRC has not been evaluated, and whether abnormal glucose metabolism could impart 5-FU resistance in CRC is also poorly defined.
方法:Three separate acquired 5-FU resistance CRC cell line models were generated, and glucose metabolism was assessed by measuring glucose and lactate utilization, RNA and protein expressions of glucose metabolism-related enzymes and changes of intermediate metabolites of glucose metabolite pool. The protein levels of hypoxia inducible factor 1α (HIF-1α) in primary tumors and circulating tumor cells of CRC patients were detected by immunohistochemistry and immunofluorescence. Stable HIF1A knockdown in cell models was established with a lentiviral system. The influence of both HIF1A gene knockdown and pharmacological inhibition on 5-FU resistance in CRC was evaluated in cell models in vivo and in vitro.
结果:The abnormality of glucose metabolism in 5-FU-resistant CRC were described in detail. The enhanced glycolysis and pentose phosphate pathway in CRC were associated with increased HIF-1α expression. HIF-1α-induced glucose metabolic reprogramming imparted 5-FU resistance in CRC. HIF-1α showed enhanced expression in 5-FU-resistant CRC cell lines and clinical specimens, and increased HIF-1α levels were associated with failure of fluorouracil analog-based chemotherapy in CRC patients and poor survival. Upregulation of HIF-1α in 5-FU-resistant CRC occurred through non-oxygen-dependent mechanisms of reactive oxygen species-mediated activation of PI3K/Akt signaling and aberrant activation of β-catenin in the nucleus. Both HIF-1α gene knock-down and pharmacological inhibition restored the sensitivity of CRC to 5-FU.
结论:HIF-1α is a potential biomarker for 5-FU-resistant CRC, and targeting HIF-1a in combination with 5-FU may represent an effective therapeutic strategy in 5-FU-resistant CRC.
关键词:HIF-1α, Metabolic reprogramming, Glycolysis, Reactive oxygen species, β-Catenin, Prognostic biomarker, 5-fluorouracil, Chemoresistance, Colorectal.