J Exp Clin Cancer Res. 2022 Jan 8.
doi: 10.1186/s13046-021-02231-y.
题目
Restriction of extracellular lipids renders pancreatic cancer dependent on autophagy
背景:KRAS is the predominant oncogene mutated in pancreatic ductal adenocarcinoma (PDAC), the fourth cause of cancer-related deaths worldwide. Mutant KRAS-driven tumors are metabolically programmed to support their growth and survival, which can be used to identify metabolic vulnerabilities. In the present study, we aimed to understand the role of extracellularly derived fatty acids in KRAS-driven pancreatic cancer.
方法:To assess the dependence of PDAC cells on extracellular fatty acids we employed delipidated serum or RNAi-mediated suppression of ACSL3 (to inhibit the activation and cellular retention of extracellular fatty acids) followed by cell proliferation assays, qPCR, apoptosis assays, immunoblots and fluorescence microscopy experiments. To assess autophagy in vivo, we employed the KrasG12D/+; p53flox/flox; Pdx1-CreERT2 (KPC) mice crossed with Acsl3 knockout mice, and to assess the efficacy of the combination therapy of ACSL3 and autophagy inhibition we used xenografted human cancer cell-derived tumors in immunocompromised mice.
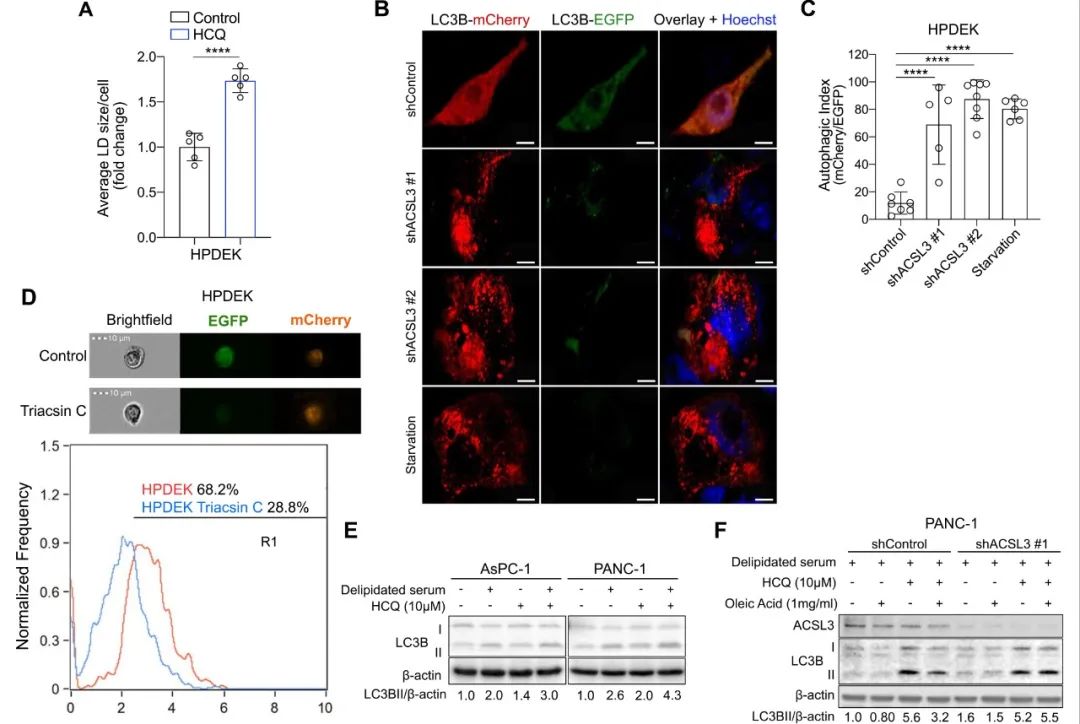
结果:Here we show that depletion of extracellularly derived lipids either by serum lipid restriction or suppression of ACSL3, triggers autophagy, a process that protects PDAC cells from the reduction of bioenergetic intermediates. Combined extracellular lipid deprivation and autophagy inhibition exhibits anti-proliferative and pro-apoptotic effects against PDAC cell lines in vitro and promotes suppression of xenografted human pancreatic cancer cell-derived tumors in mice. Therefore, we propose lipid deprivation and autophagy blockade as a potential co-targeting strategy for PDAC treatment.
结论:Our work unravels a central role of extracellular lipid supply in ensuring fatty acid provision in cancer cells, unmasking a previously unappreciated metabolic vulnerability of PDAC cells.
关键词:Pancreatic cancer, Lipid metabolism, Extracellular lipids, Combination therapy, Tumor metabolic vulnerabilities

