J Exp Clin Cancer Res. 2022 Jan 15.
doi: 10.1186/s13046-021-02235-8.
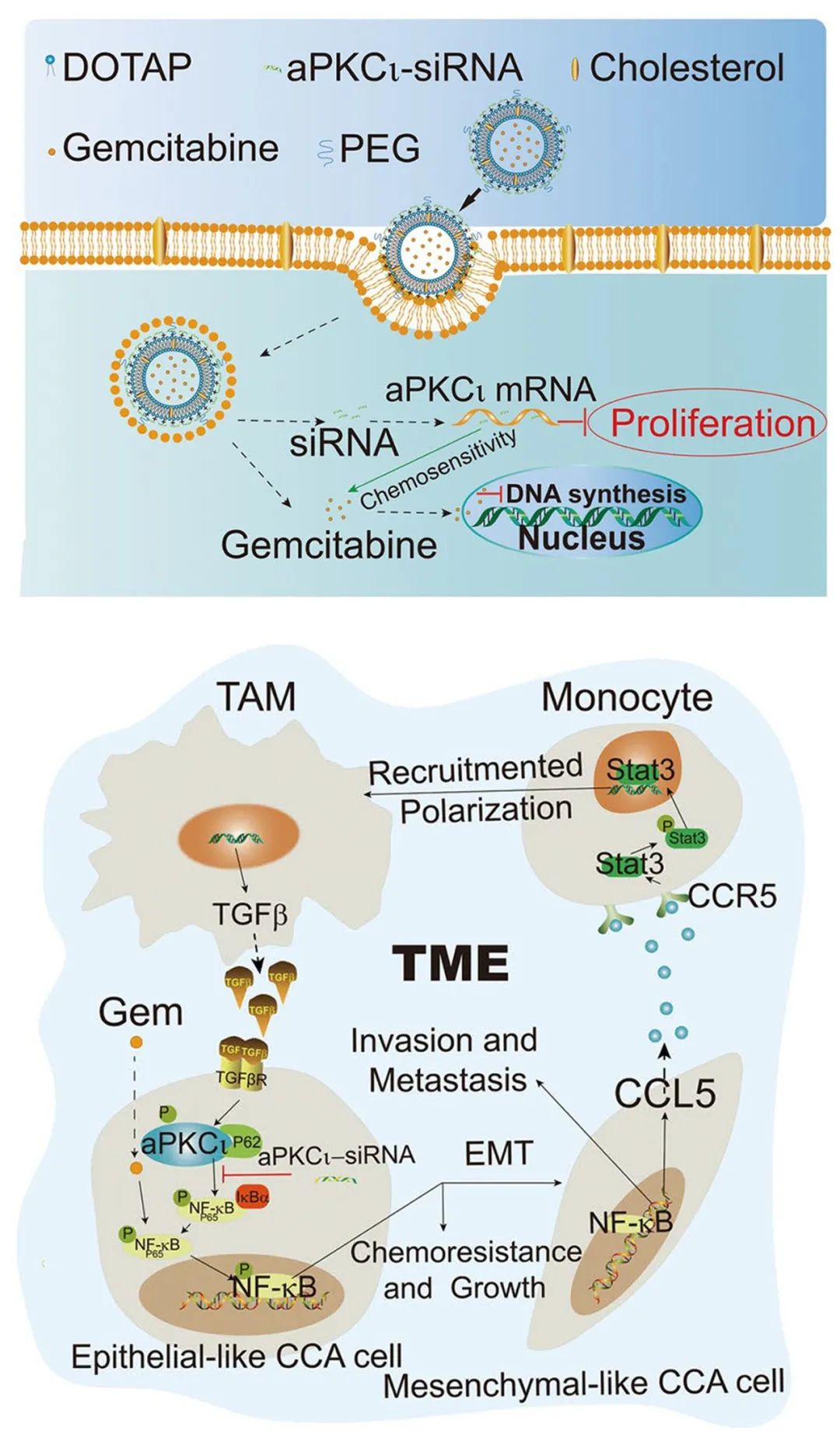
Macrophages-aPKCι-CCL5 Feedback Loop Modulates the Progression and Chemoresistance in Cholangiocarcinoma
背景:Recent data indicated that macrophages may mutually interact with cancer cells to promote tumor progression and chemoresistance, but the interaction in cholangiocarcinoma (CCA) is obscure.
方法:10x Genomics single-cell sequencing technology was used to identified the role of macrophages in CCA. Then, we measured the expression and prognostic role of macrophage markers and aPKCι in 70 human CCA tissues. Moreover, we constructed monocyte-derived macrophages (MDMs) generated from peripheral blood monocytes (PBMCs) and polarized them into M1/M2 macrophages. A co-culture assay of the human CCA cell lines (TFK-1, EGI-1) and differentiated PBMCs-macrophages was established, and functional studies in vitro and in vivo was performed to explore the interaction between cancer cells and M2 macrophages. Furthermore, we established the cationic lipo-some-mediated co-delivery of gemcitabine and aPKCι-siRNA and detect the antitumor effects in CCA.
结果:M2 macrophage showed tumor-promoting properties in CCA. High levels of aPKCι expression and M2 mac-rophage infiltration were associated with metastasis and poor prognosis in CCA patients. Moreover, CCA patients with low M2 macrophages infiltration or low aPKCι expression benefited from postoperative gemcitabine-based chemo-therapy. Further studies showed that M2 macrophages-derived TGFβ1 induced epithelial-mesenchymal transition (EMT) and gemcitabine resistance in CCA cells through aPKCι-mediated NF-κB signaling pathway. Reciprocally, CCL5 was secreted more by CCA cells undergoing aPKCι-induced EMT and consequently modulated macrophage recruit-ment and polarization. Furthermore, the cationic liposome-mediated co-delivery of GEM and aPKCι-siRNA signifi-cantly inhibited macrophages infiltration and CCA progression.
结论:our study demonstrates the role of Macrophages-aPKCι-CCL5 Feedback Loop in CCA, and proposes a novel therapeutic strategy of aPKCι-siRNA and GEM co-delivered by liposomes for CCA.
关键词:Cholangiocarcinoma, Positive Feedback Loop, Macrophages, aPKCι, Chemoresistance

