doi: 10.1186/s13073-021-01002-w.
背景:N6-methyladenosine (m6A) is the most abundant modification of RNA in eukaryotic cells and play critical roles in cancer. While most related studies focus on m6A modifications in linear RNA, transcriptome-wide profiling and exploration of m6A modification in circular RNAs in cancer is still lacking.
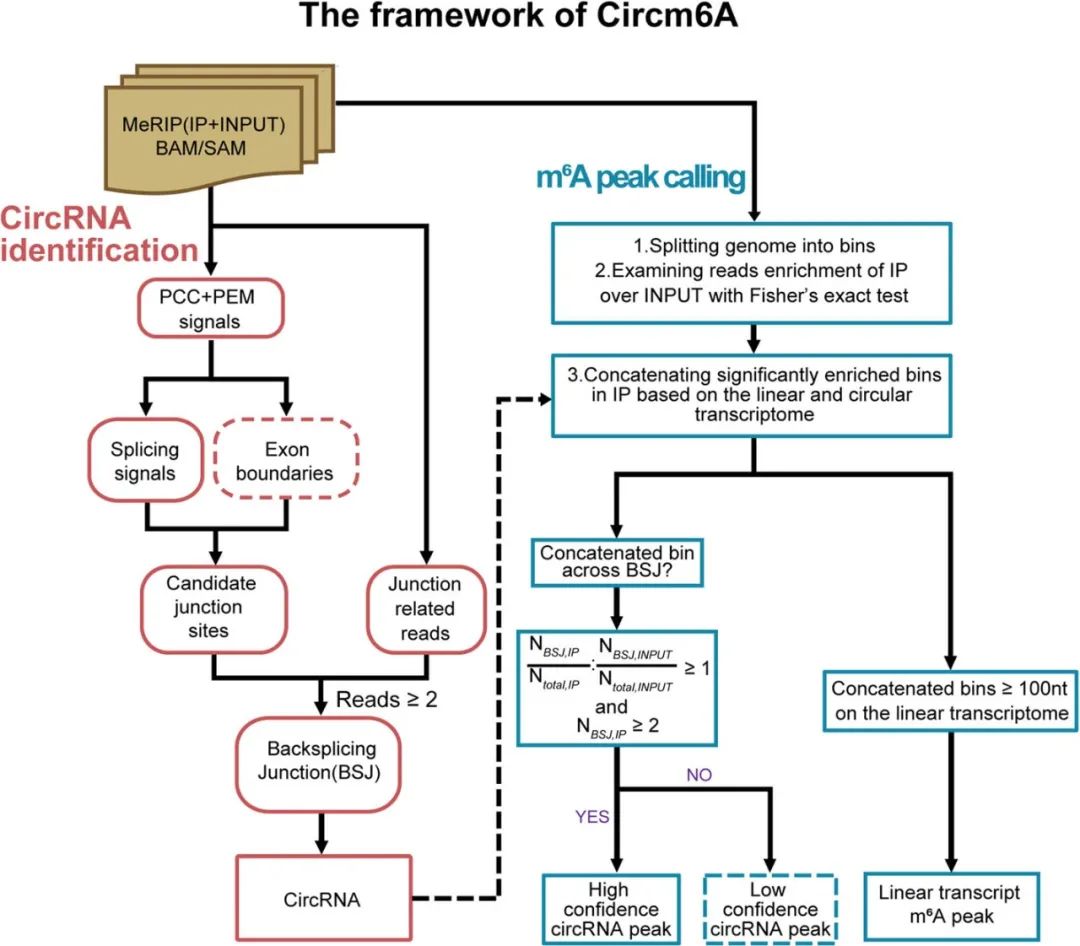
方法:For the detection of m6A modification in circRNAs, we developed a new bioinformatics tools called Circm6A and applied it to the m6A-seq data of 77 tissue samples from 58 individuals with pancreatic ductal adenocarcinoma (PDAC).
结果:Circm6A performs better than the existing circRNA identification tools, which achieved highest F1 score among these tools in the detection of circRNAs with m6A modifications. By using Circm6A, we identified a total of 8807 m6A-circRNAs from our m6A-seq data. The m6A-circRNAs tend to be hypermethylated in PDAC tumor tissues compared with normal tissues. The hypermethylated m6A-circRNAs were associated with a significant gain of circRNA-mRNA coexpression network, leading to the dysregulation of many important cancer-related pathways. Moreover, we found the cues that hypermethylated m6A-circRNAs may promote the circularization and translation of circRNAs.
结论:These comprehensive findings further bridged the knowledge gaps between m6A modification and circRNAs fields by depicting the m6A-circRNAs genomic landscape of PDAC patients and revealed the emerging roles played by m6A-circRNAs in pancreatic cancer. Circm6A is available at https://github.com/canceromics/circm6a.
关键词:m6A modification, Circular RNA, m6A-seq, Pancreatic cancer

