期刊及DOI号
Downregulation of PHLPP induced by endoplasmic reticulum stress promotes eIF2α phosphorylation and chemoresistance in colon cancer
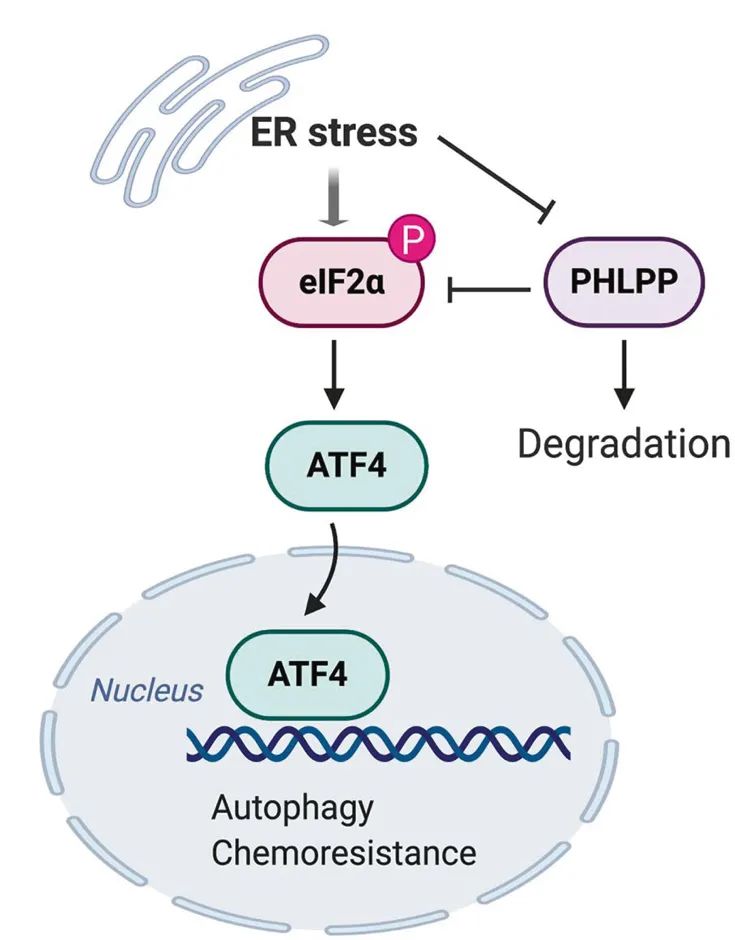
Aberrant activation of endoplasmic reticulum (ER) stress by extrinsic and intrinsic factors contributes to tumorigenesis and resistance to chemotherapies in various cancer types. Our previous studies have shown that the downregulation of PHLPP, a novel family of Ser/Thr protein phosphatases, promotes tumor initiation, and progression. Here we investigated the functional interaction between the ER stress and PHLPP expression in colon cancer. We found that induction of ER stress significantly decreased the expression of PHLPP proteins through a proteasome-dependent mechanism. Knockdown of PHLPP increased the phosphorylation of eIF2α as well as the expression of autophagy-associated genes downstream of the eIF2α/ATF4 signaling pathway. In addition, results from immunoprecipitation experiments showed that PHLPP interacted with eIF2α and this interaction was enhanced by ER stress. Functionally, knockdown of PHLPP improved cell survival under ER stress conditions, whereas overexpression of a degradation-resistant mutant PHLPP1 had the opposite effect. Taken together, our studies identified ER stress as a novel mechanism that triggers PHLPP downregulation; and PHLPP-loss promotes chemoresistance by upregulating the eIF2α/ATF4 signaling axis in colon cancer cells.

