Brain Pathol. 2021 Nov 29.
Lumbar spine intrathecal transplantation of neural precursor cells promotes oligodendrocyte proliferation in hot spots of chronic demyelination
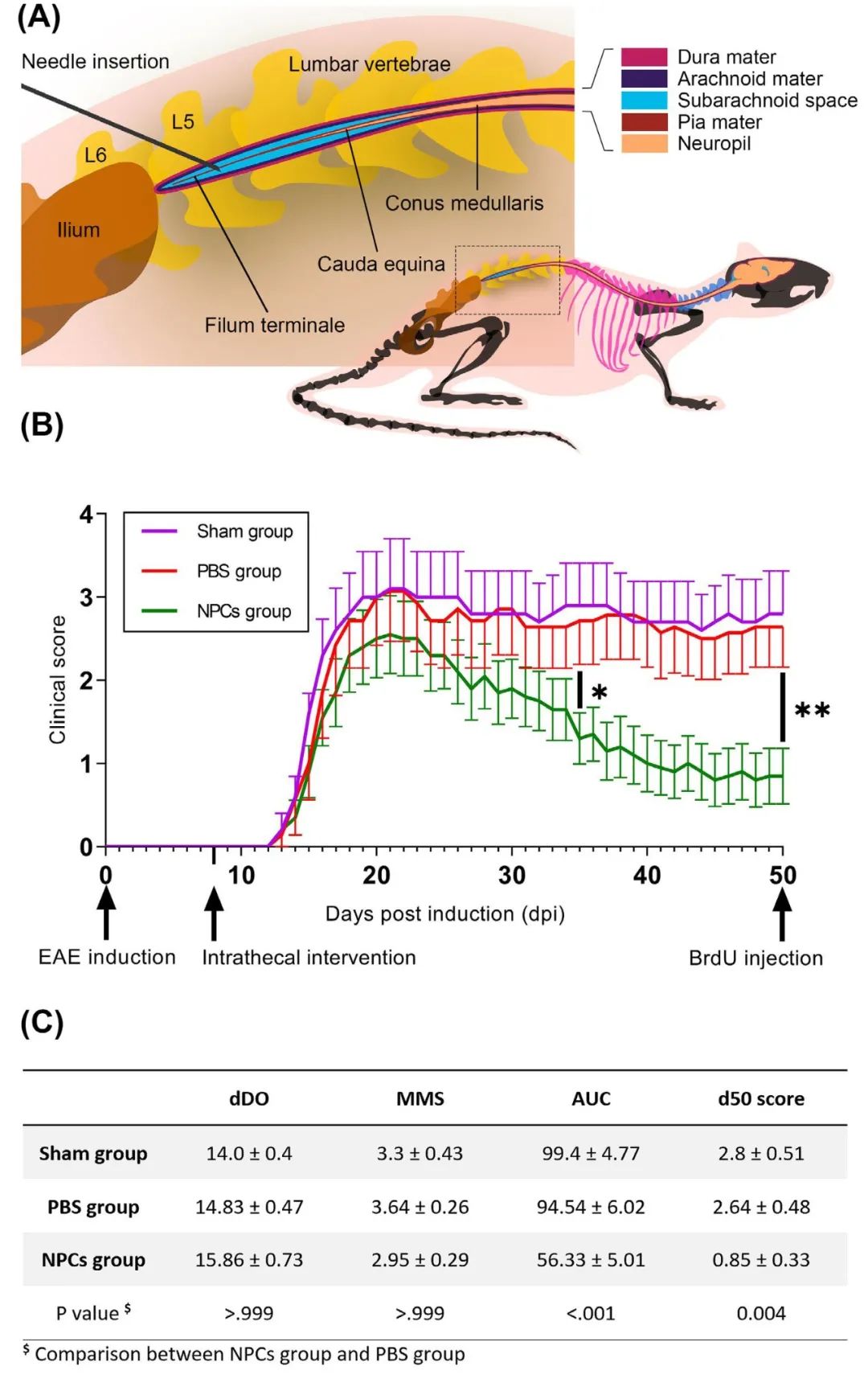
Experimental autoimmune encephalomyelitis (EAE) is a basic and reliable model used to study clinical and pathological hallmarks of multiple sclerosis (MS) in rodents. Several studies suggest neural precursor cells (NPCs) as a significant research tool while reporting that transplanted NPCs are a promising therapeutic approach to treating neurological disorders, such as MS. The main objective was to approach a preclinical, in vivo scenario of oligodendrogenesis with NPCs, targeting the main chronic demyelinated lumbosacral milieu of EAE, via the least invasive delivery method which is lumbar puncture. We utilized MOG35-55 peptide to induce EAE in C57BL/6 mice and prior to the acute relapse, we intervened with either the traceable GFP+ cellular therapy or saline solution in the intrathecal space of their lumbar spine. A BrdU injection, which enabled us to monitor endogenous proliferation, marked the endpoint 50 days post-induction (50 dpi). Neuropathology with high-throughput, triple immunofluorescent, and transmission electron microscopy (TEM) data were extracted and analyzed. The experimental treatment attenuated the chronic phase of EAE (50 dpi; score <1) following an acute, clinical relapse. Myelination and axonal integrity were rescued in the NPC-treated animals along with suppressed immune populations. The differentiation profile of the exogenous NPCs and endogenous BrdU+ cells was location-dependent where GFP+-rich areas drove undifferentiated phenotypes toward the oligodendrocyte lineage. In situ oligodendrocyte enrichment was demonstrated through increased (p<0.001) gap junction channels of Cx32 and Cx47, reliable markers for proliferative oligodendroglia syncytium. TEM morphometric analysis ultimately manifested an increased g-ratio in lumbosacral fibers of the recovered animals (p<0.001). Herein, we suggest that a single, lumbar intrathecal administration of NPCs capacitated a viable cellular load and resulted in clinical and pathological amelioration, stimulating resident OPCs to overcome the remyelination failure in EAE demyelinating locale.