题目
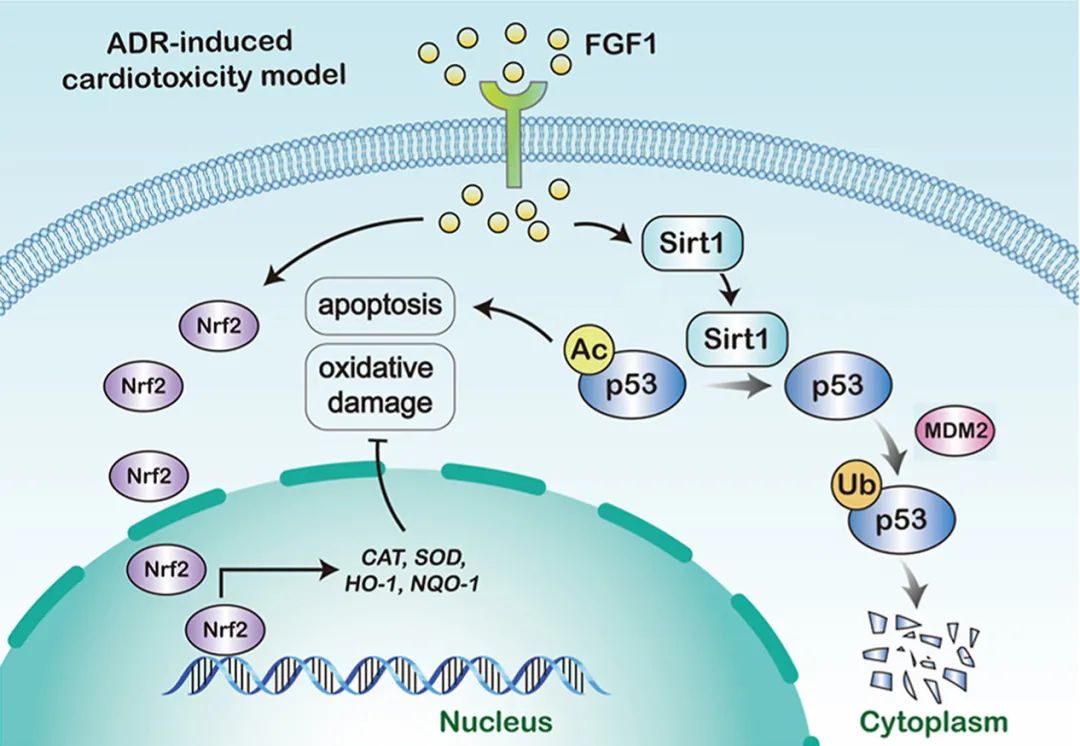
A cumulative and progressively developing cardiomyopathy induced by adriamycin (ADR)-based chemotherapy is a major obstacle for its clinical application. However, there is a lack of safe and effective method to protect against ADR-induced cardiotoxicity. Here, we found that mRNA and protein levels of FGF1 were decreased in ADR-treated mice, primary cardiomyocytes and H9c2 cells, suggesting the potential effect of FGF1 to protect against ADR-induced cardiotoxicity. Then, we showed that treatment with a FGF1 variant (FGF1ΔHBS) with reduced proliferative potency significantly prevented ADR-induced cardiac dysfunction as well as ADR-associated cardiac inflammation, fibrosis, and hypertrophy. The mechanistic study revealed that apoptosis and oxidative stress, the two vital pathological factors in ADR-induced cardiotoxicity, were largely alleviated by FGF1ΔHBS treatment. Furthermore, the inhibitory effects of FGF1ΔHBS on ADR-induced apoptosis and oxidative stress were regulated by decreasing p53 activity through upregulation of Sirt1-mediated p53 deacetylation and enhancement of murine double minute 2 (MDM2)-mediated p53 ubiquitination. Upregulation of p53 expression or cardiac specific-Sirt1 knockout (Sirt1-CKO) almost completely abolished FGF1ΔHBS-induced protective effects in cardiomyocytes. Based on these findings, we suggest that FGF1ΔHBS may be a potential therapeutic agent against ADR-induced cardiotoxicity.

