2022年1月15日,上海交通大学医学院附属瑞金医院妇产科冯炜炜团队在Journal of Experimental and Clinical Cancer Research上发表了题为“Genome-wide CRISPR/Cas9 library screen identifies PCMT1 as a critical driver of ovarian cancer metastasis”的研究论文。在本研究中,团队通过系统识别抗失巢凋亡的驱动因素,揭示了PCMT1对粘着斑(FA)动力学以及癌症转移的贡献,研究表明PCMT1可能成为转移性卵巢癌的治疗靶点。
肿瘤转移是一个多步骤、高度选择性的过程,在此过程中,肿瘤细胞处于动态状态,并且容易因ECM的分离而发生失巢凋亡。从原发部位脱落的卵巢癌细胞能够在体内单个细胞或腹水中自由漂浮的团块中存活,这可能就是抗失巢凋亡的结果。
肿瘤细胞可分泌大量ECM成分,它们在转移性肿瘤中高度表达,有助于建立肿瘤转移生态位。除了众所周知的结构支架功能外,ECM成分还位于细胞之间,并介导细胞间相互作用。ECM在肿瘤转移进展中的作用,尤其是沉积、翻译后修饰和重组以及与癌细胞表面受体的动态相互作用,都受到生物化学和机械信号的严格调控。
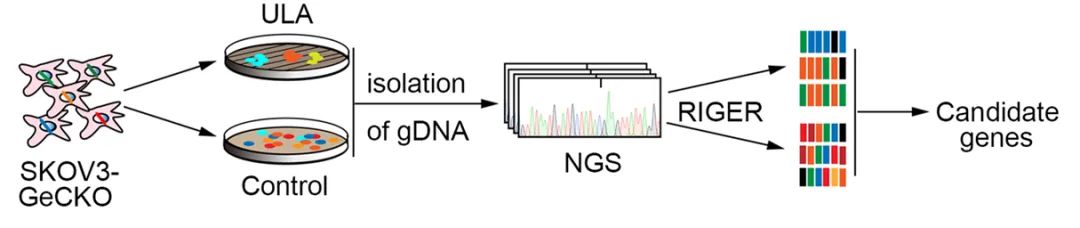
为了鉴定抗失巢凋亡和癌症进展所需的可能的治疗靶点蛋白,团队在卵巢癌细胞中进行了全基因组CRISPR/cas9基因敲除筛选,以模拟癌细胞从原始附着点脱落的情况。结果发现PCMT1基因与卵巢癌的抗失巢凋亡最高度相关。
在此项研究中,团队将PCMT1鉴定为蛋白质修复中的一种关键酶,是癌症抗失巢凋亡的关键驱动因素。重要的是,团队首先将PCMT1与ECM蛋白LAMB3联系起来,并证明PCMT1-LAMB3相互作用可激活整合素-FAK-Src信号通路,进而促进癌细胞粘附、迁移和侵袭,并且这些作用可通过PCMT1阻断抗体治疗来逆转。此外,PCMT1过表达增强了异种移植小鼠模型的腹水形成和远处转移,一致的,PCMT1的敲除具有相反的效果。临床上,与早期原发性肿瘤样本相比,PCMT1在晚期转移性肿瘤样本中高度表达。
图 在正常单层培养和超低粘附(ULA)平板中,全基因组CRISPR筛选示意图。
J Exp Clin Cancer Res. 2022 Jan 15.
doi: 10.1186/s13046-022-02242-3.
Genome-wide CRISPR/Cas9 library screen identifies PCMT1 as a critical driver of ovarian cancer metastasis
背景:The development of lethal cancer metastasis depends on the dynamic interactions between cancer cells and the tumor microenvironment, both of which are embedded in the extracellular matrix (ECM). The acquisition of resistance to detachment-induced apoptosis, also known as anoikis, is a critical step in the metastatic cascade. Thus, a more in-depth and systematic analysis is needed to identify the key drivers of anoikis resistance.
方法:Genome-wide CRISPR/Cas9 knockout screen was used to identify critical drivers of anoikis resistance using SKOV3 cell line and found protein-L-isoaspartate (D-aspartate) O-methyltransferase (PCMT1) as a candidate. Quantitative real-time PCR (qRT-PCR) and immune-histochemistry (IHC) were used to measure differentially expressed PCMT1 in primary tissues and metastatic cancer tissues. PCMT1 knockdown/knockout and overexpression were performed to investigate the functional role of PCMT1 in vitro and in vivo. The expression and regulation of PCMT1 and integrin-FAK-Src pathway were evaluated using immunoprecipitation followed by mass spectrometry (IP-MS), western blot analysis and live cell imaging.
结果:We found that PCMT1 enhanced cell migration, adhesion, and spheroid formation in vitro. Interestingly, PCMT1 was released from ovarian cancer cells, and interacted with the ECM protein LAMB3, which binds to integrin and activates FAK-Src signaling to promote cancer progression. Strikingly, treatment with an antibody against extracellular PCMT1 effectively reduced ovarian cancer cell invasion and adhesion. Our in vivo results indicated that overexpression of PCMT1 led to increased ascites formation and distant metastasis, whereas knockout of PCMT1 had the opposite effect. Importantly, PCMT1 was highly expressed in late-stage metastatic tumors compared to early-stage primary tumors.
结论:Through systematically identifying the drivers of anoikis resistance, we uncovered the contribution of PCMT1 to focal adhesion (FA) dynamics as well as cancer metastasis. Our study suggested that PCMT1 has the potential to be a therapeutic target in metastatic ovarian cancer.
关键词:PCMT1, Metastasis, Extracellular matrix, CRISPR/Cas9, Integrin-FAK-Src, Anoikis