题目
FasL+ PD-L2+ Identifies a Novel Immunosuppressive Neutrophil Population in Human Gastric Cancer That Promotes Disease Progression
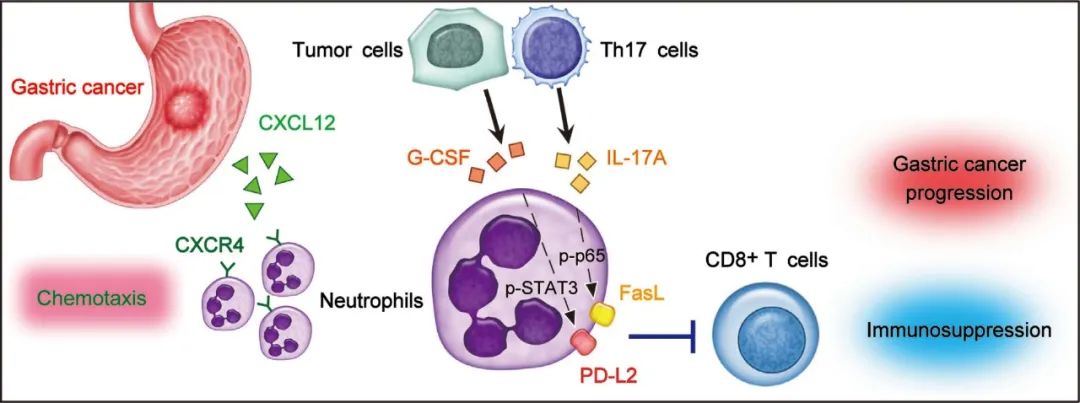
Neutrophils constitute abundant cellular components in human gastric cancer (GC) tissues, but their protumorigenic subset in pathogenesis of GC progression is unclear. Here, it is found that patients with GC show significantly higher neutrophil infiltration in tumors that is regulated by CXCL12-CXCR4 chemotaxis. These tumor-infiltrating neutrophils express high level immunosuppressive molecules FasL and PD-L2, and this FasL+PD-L2+ neutrophil subset with a unique phenotype constitutes at least 20% of all neutrophils in advanced GC and predicts poor patient survival. Tumor induces neutrophils to express FasL and PD-L2 proteins with similar phenotype to those in GC tumors in both time-dependent and dose-dependent manners. Mechanistically, Th17 cell-derived IL-17A and tumor cell-derived G-CSF can significantly induce neutrophil FasL and PD-L2 expression via activating ERK-NF-κB and JAK-STAT3 signaling pathway, respectively. Importantly, upon over-expressing FasL and PD-L2, neutrophils acquire immunosuppressive functions on tumor-specific CD8+ T-cells and promote the growth and progression of human GC tumors in vitro and in vivo, which can be reversed by blocking FasL and PD-L2 on these neutrophils. Thus, the work identifies a novel protumorigenic FasL+PD-L2+ neutrophil subset in GC and provides new insights for human cancer immunosuppression and anti-cancer therapies targeting these pathogenic cells.

